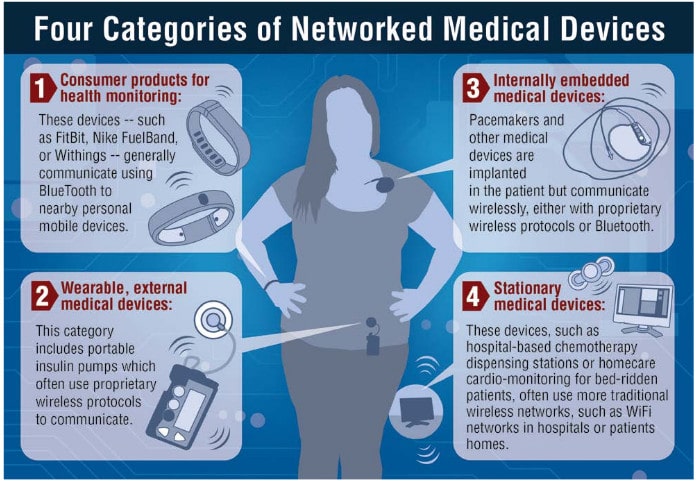
In-brief: the U.S. healthcare sector is in critical condition and needs urgent, coordinated action to protect patient safety and address vulnerabilities in millions of deployed medical devices, a Congressional Task Force has concluded. (Updated with comments from Joshua Corman of Atlantic Council. PFR June 7, 2017)
FDA Workshop
FDA: Medical Device Makers Urged To Secure Post Market Devices from Cyber Attack
In-brief: The U.S. Food and Drug Administration (FDA) on Friday issued new guidelines, calling on medical device makers to do a better job addressing cyber security vulnerabilities and exploits as part of their management of deployed medical devices.
Could hackers break my heart via my pacemaker? – BBC News
In-brief: A BBC article profiles an information security expert who finds herself the bearer of an implanted pacemaker, raising issues about the risks and benefits of new, connected health devices.
FDA Seeks Collaboration on Medical Device Security
The U.S. Food and Drug Administration (FDA) on Tuesday put out a call for ideas and input on how best to secure medical devices and the healthcare system from cyber attack. In a federal notice, the FDA announced that it will hold an October workshop entitled “Collaborative Approaches for Medical Device and Healthcare Cybersecurity.” It also solicited input from stakeholders within the government and from the public health sector on medical device and healthcare cyber security. The workshop is scheduled for October 21 and 22 and will run from 9:00 AM to 5:00PM at the National Intellectual Property Rights Coordination Center Auditorium in Arlington, Virginia. [Read more Security Ledger coverage of connected medical devices here.] The Department of Health and Human Services (HHS) is looking for ideas about how best to implement aspects of both Executive Order 13636 for“Improving Critical Infrastructure” and follow-on guidance like the National Institute of Standards and Technology’s (NIST’s) “Framework for Improving […]