Cybersecurity teams are turning to artificial intelligence to cover a gap in skilled cyber talent, a report from Code42 finds.
Pharmaceutical
NotPetya Infection Left Merck Short of Key HPV Vaccine
The NotPetya malware infection shut down pharmaceutical giant Merck’s production of the pediatric vaccine GARDASIL last June, forcing the company to borrow the drug from a stockpile maintained by the U.S. Centers for Disease Control and Prevention to meet demand.
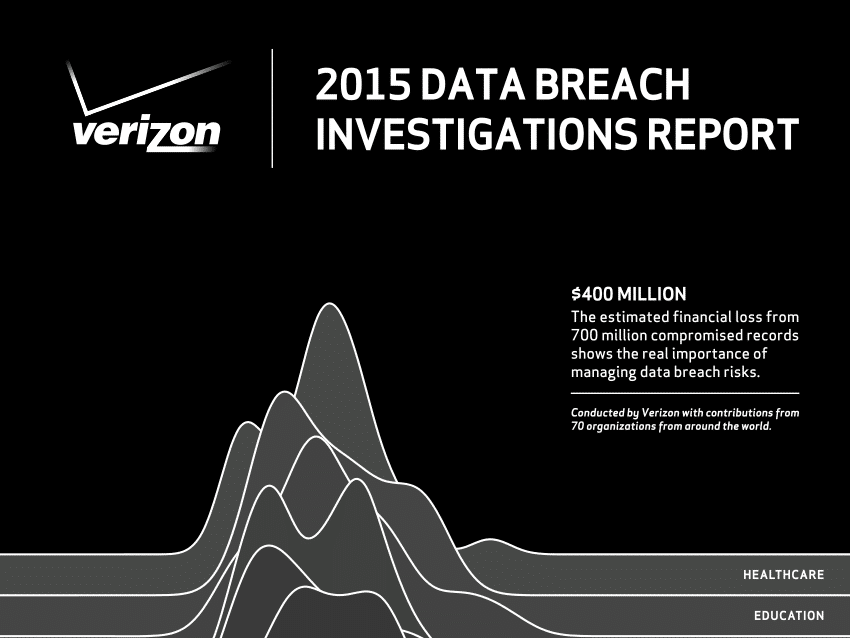
Verizon: Internet of Things Hacks Pose Little Risk – For Now
In-brief: Verizon said in its latest Data Breach Investigations Report that threats from Internet of Things technologies were more theory than practice in 2014, but that 2015 could see IoT devices play a role in breaches.
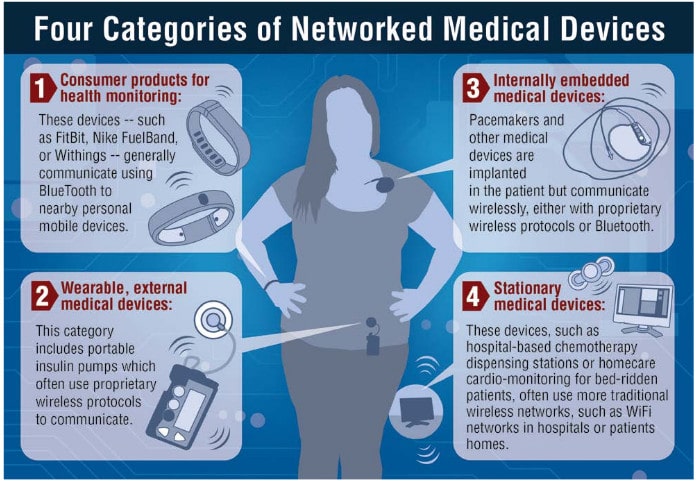
Intel: New Approach Needed to Secure Connected Health Devices
In-brief: connected medical devices pose a number of risks to patients, including the threat of “targeted killings,” according to a report by Intel Security. The fix: better application design and more public-private sector cooperation.
FDA Seeks Collaboration on Medical Device Security
The U.S. Food and Drug Administration (FDA) on Tuesday put out a call for ideas and input on how best to secure medical devices and the healthcare system from cyber attack. In a federal notice, the FDA announced that it will hold an October workshop entitled “Collaborative Approaches for Medical Device and Healthcare Cybersecurity.” It also solicited input from stakeholders within the government and from the public health sector on medical device and healthcare cyber security. The workshop is scheduled for October 21 and 22 and will run from 9:00 AM to 5:00PM at the National Intellectual Property Rights Coordination Center Auditorium in Arlington, Virginia. [Read more Security Ledger coverage of connected medical devices here.] The Department of Health and Human Services (HHS) is looking for ideas about how best to implement aspects of both Executive Order 13636 for“Improving Critical Infrastructure” and follow-on guidance like the National Institute of Standards and Technology’s (NIST’s) “Framework for Improving […]