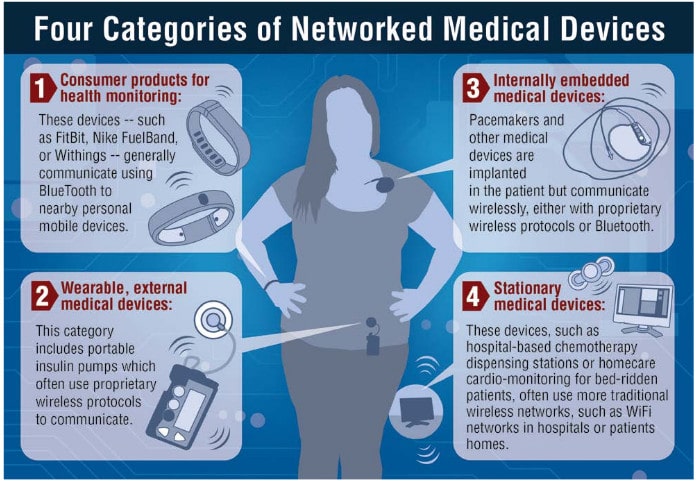
In-brief: A BBC article profiles an information security expert who finds herself the bearer of an implanted pacemaker, raising issues about the risks and benefits of new, connected health devices.
HHS
Clueless Clause: Insurer Cites Lax Security in Challenge to Cottage Health Claim
In-brief: In what may become a trend, an insurance company is denying a claim from a California healthcare provider following the leak of data on more than 32,000 patients. The insurer, Columbia Casualty, charges that Cottage Health System did an inadequate job of protecting patient data.
Researcher: Drug Pump the ‘Least Secure IP Device I’ve Ever Seen’
In-brief: A researcher studying the workings of a wireless-enabled drug infusion pump by the firm Hospira said the device utterly lacked security controls, making it “the least secure IP enabled device” he had ever worked with. His research prompted a warning from the Department of Homeland Security.
FDA Issues Guidance on Security of Medical Devices
The U.S. Food and Drug Administration (FDA) issued final guidance on Wednesday that are designed to strengthen the safety of medical devices. The FDA called on medical device manufacturers to consider cyber security risks as part of the design and development of devices. The document, “Content of Premarket Submissions for Management of Cybersecurity in Medical Devices,” asks device makers to submit documentation to the FDA about any “risks identified and controls in place to mitigate those risks” in medical devices. The guidance also recommends that manufacturers submit documentation of plans for patching and updating the operating systems and medical software that devices run. The document, which will be released on Thursday, does not contain specific requirements. Rather, it describes the kinds of things that medical device manufacturers should consider when preparing pre-market submissions for medical devices in areas such as information confidentiality, integrity, and availability, the FDA said. The release of the document follows the […]
FDA Seeks Collaboration on Medical Device Security
The U.S. Food and Drug Administration (FDA) on Tuesday put out a call for ideas and input on how best to secure medical devices and the healthcare system from cyber attack. In a federal notice, the FDA announced that it will hold an October workshop entitled “Collaborative Approaches for Medical Device and Healthcare Cybersecurity.” It also solicited input from stakeholders within the government and from the public health sector on medical device and healthcare cyber security. The workshop is scheduled for October 21 and 22 and will run from 9:00 AM to 5:00PM at the National Intellectual Property Rights Coordination Center Auditorium in Arlington, Virginia. [Read more Security Ledger coverage of connected medical devices here.] The Department of Health and Human Services (HHS) is looking for ideas about how best to implement aspects of both Executive Order 13636 for“Improving Critical Infrastructure” and follow-on guidance like the National Institute of Standards and Technology’s (NIST’s) “Framework for Improving […]