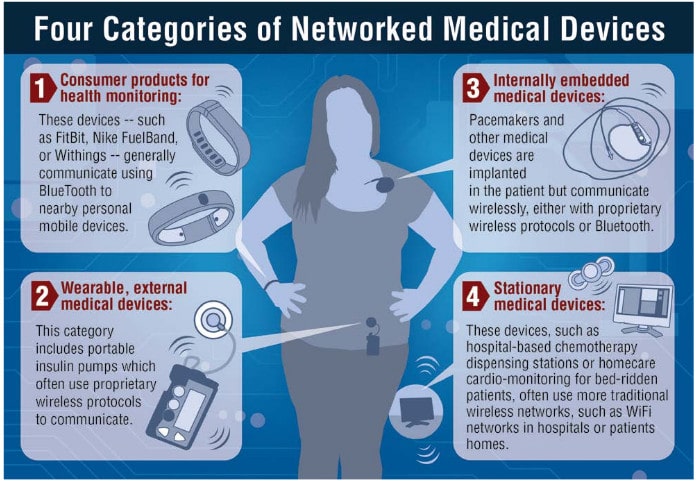
In-brief: A prominent expert in medical device security has warned the FDA that its draft guidance for post market medical devices is too focused on stomping out known threats, and not enough on addressing current and future risks to the security of healthcare environments.
FDA
NHTSA Drafting Cyber Security Guidelines for Light Vehicles
In-brief: The National Highway Traffic and Safety Administration (NHTSA) is drafting cyber security guidelines for light vehicles, the agency’s first major initiative exclusively focused on the security of connected cars.
FDA: Medical Device Makers Urged To Secure Post Market Devices from Cyber Attack
In-brief: The U.S. Food and Drug Administration (FDA) on Friday issued new guidelines, calling on medical device makers to do a better job addressing cyber security vulnerabilities and exploits as part of their management of deployed medical devices.
Could hackers break my heart via my pacemaker? – BBC News
In-brief: A BBC article profiles an information security expert who finds herself the bearer of an implanted pacemaker, raising issues about the risks and benefits of new, connected health devices.
Epidemic: Researchers Find Thousands of Medical Systems Exposed to Hackers
In-brief: Thousands of clinical systems are exposed to remote attacks according to researchers, who say that poorly designed and loosely configured medical devices are a major source of insecurity.