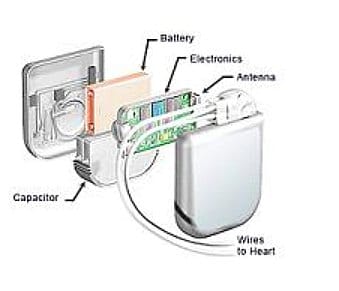
In this industry perspective, Dan Lyon and Taylor Armerding of the firm Synopsys discuss the impact of the FDA’s new Medical Device Safety Action Plan, which promises to improve the cyber security of medical devices…eventually.
FDA
A Year Later: FDA approves Software Fix for Security Flaws in Pacemakers
In-brief: The FDA as approved a software update to software security holes in pacemakers made by Abbott. But doctors and patients will have to weigh the risks of apply the patch.
Update: Cash for Medical Device Clunkers? Task Force calls for Healthcare Security Overhaul
In-brief: the U.S. healthcare sector is in critical condition and needs urgent, coordinated action to protect patient safety and address vulnerabilities in millions of deployed medical devices, a Congressional Task Force has concluded. (Updated with comments from Joshua Corman of Atlantic Council. PFR June 7, 2017)
Code Blue: 8k Vulnerabilities in Software to manage Cardiac Devices
Software used to remotely program implantable cardiac devices by a number of vendors is rife with exploitable software vulnerabilities that leave the devices vulnerable to attacks and compromise, according to a report by the firm Whitescope Inc.
Update: FDA says St. Jude Medical knew about Device Flaws 2 Years Before Muddy Waters Report
In-brief: In a damning report, the FDA said that St. Jude Medical* knew about serious security flaws in its implantable medical devices as early as 2014, but failed to address them with software updates or other mitigations, or by replacing those devices. (Editor’s note: updated to include a statement from Abbott and comment from Dr. Kevin Fu. – PFR April 14, 2017)